Pushing the boundaries of care
INVU by Nuvo™ provides peace of mind by enabling remote fetal monitoring
from the convenience of your home or work


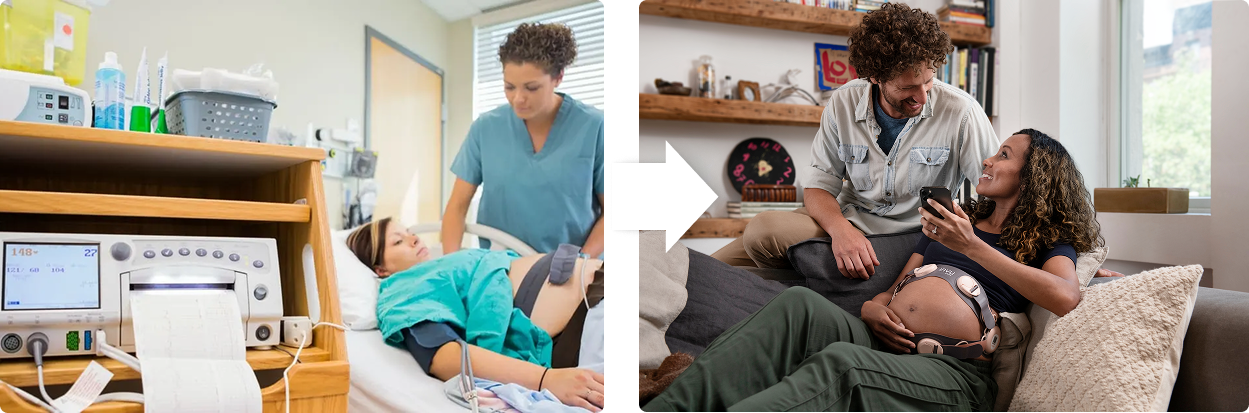
Legacy Care
Antiquated technology in resource intensive clinical settings
Next Gen Care
Nuvo enables data-driven, actionable insights, anywhere, by everyone.
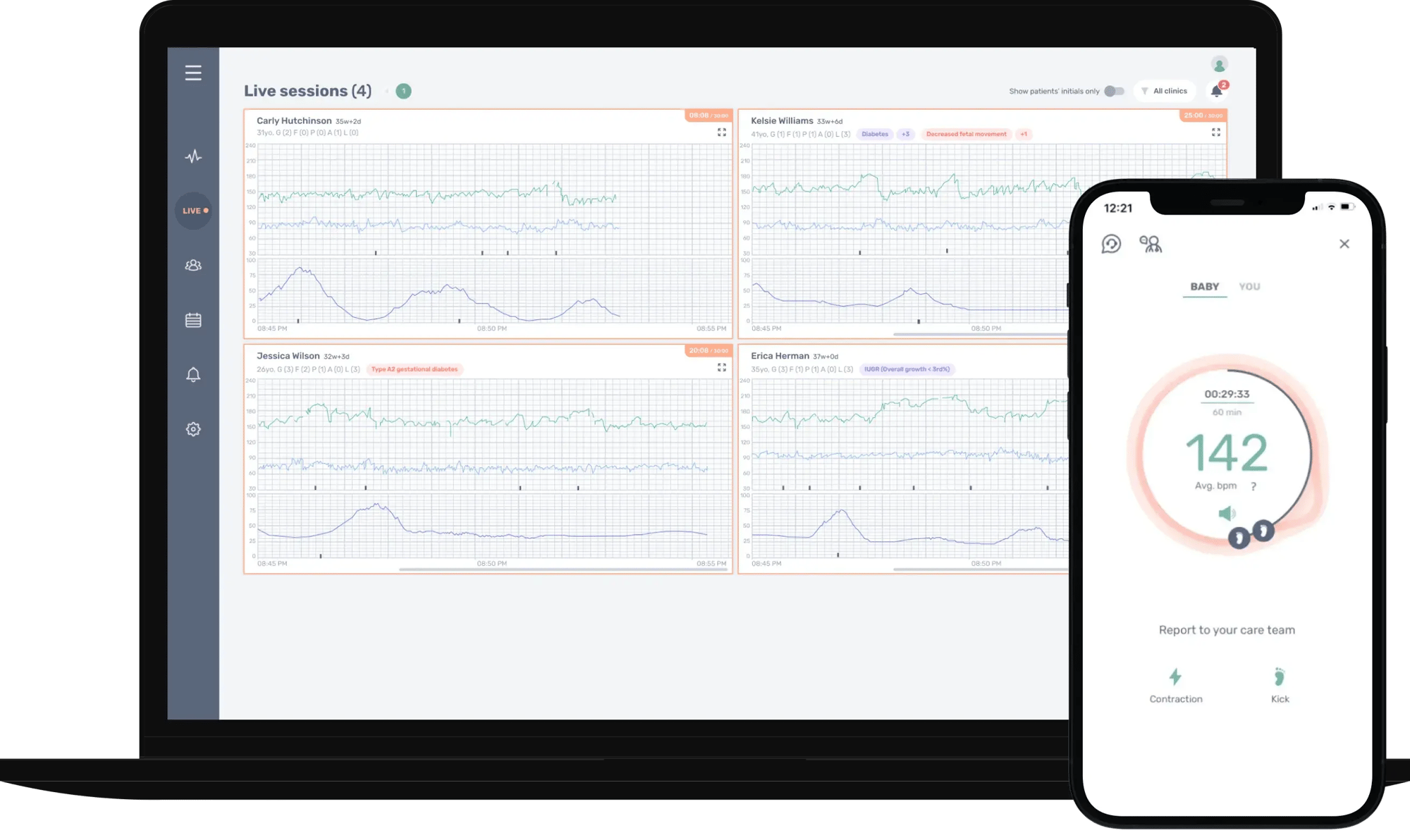
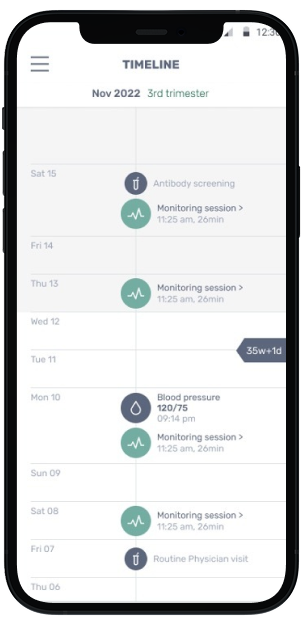
Manage multiple remote fetal monitoring sessionsat the same time
Mothers see useful, but not overly-detailed, information.


Nuvo tech support team is monitoring every session so clinicians don’t have to do tech troubleshooting

Remote fetal monitoring equipment and training fully managed by Nuvo

OB prescribes INVU™
OB prescribe INVU Sensor Band, and blood pressure (“BP”) cuff if necessary

Nuvo shipment
Nuvo ships device(s) to patient
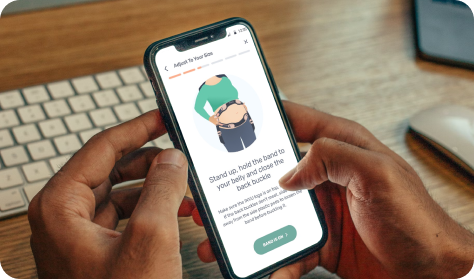
Downloads app
Mother downloads app and registers device(s)

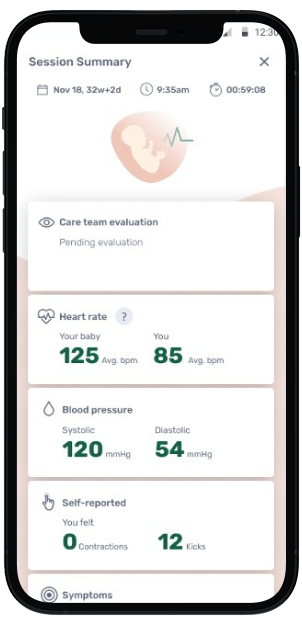
Mother's View
Mother views meaningful data in INVU app
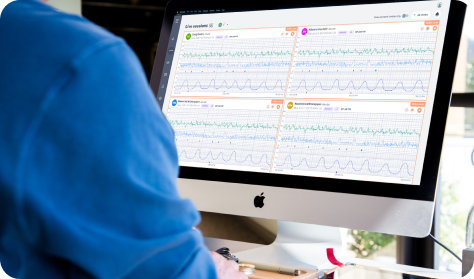
Clinician view
Provider sees comprehensive data through Provider Dashboard

Mother return
Mother returns device(s) to Nuvo
Extensive research & clinical validation
Mothers and their physicians love INVU by Nuvo
Patents & Certifications
17
Core U.S. patents
43
Granted Patents Worldwide
25
Additional Patents Pending



INVU™ is cleared by the FDA for fetal heart rate, maternal heart rate, and uterine activity (contractions). INVU™ is HIPPA compliant and certified ISO 13485 and 27001.